Restalyne for the Lips
July 7, 2018Xeomin Antiwrinkle
July 7, 2018Restylane Lyft Approved to Correct Volume Deficit in the Dorsal Hand

The Food and Drug Administration (FDA) has approved Restylane Lyft with Lidocaine (hyaluronic acid [HA] dermal filler) for injection into the subcutaneous plane in the dorsal hand to correct volume deficit in patients ?21 years old.
The approval was based on a multicenter, randomized, evaluator-blinded, split-hand study (N=89) which showed that treatment with Restylane Lyft resulted in a clinically meaningful improvement in the correction of volume deficits of treated hands for up to 6 months. With regard to adverse effects, injection site responses, including swelling, tenderness, bruising, itching and impaired hand function, were observed during the study but were generally considered mild in intensity and temporary.
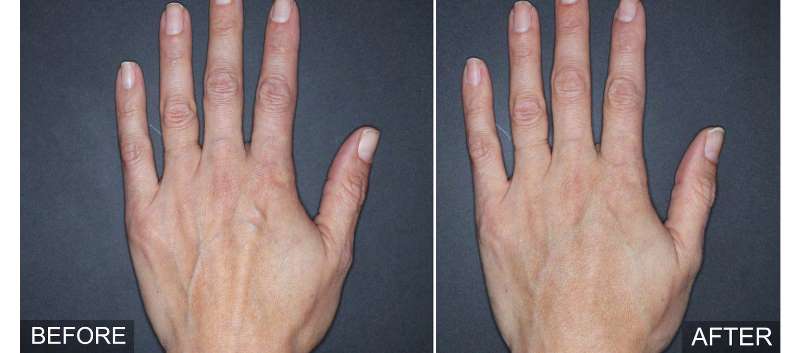
“Restylane Lyft offers a safe and effective HA treatment option for restoring volume to the hands and delivering natural-looking results,” added Dr. David Bank, New York dermatologist and clinical investigator for Restylane Lyft in the hands.
Restylane Lyft with Lidocaine is also indicated for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation for the correction of age-related midface contour deficiencies in patients ?21 years old.
https://www.empr.com/news/hyaluronic-acid-restylane-lyft-dorsal-hand-aging-volume-deficit/article/767277/

